LYNNWOOD, Was., December 5, 2021 – As Reuters reported in November, a group of scientists issued a Freedom of Information Act (FOIA) request, asking the U.S. Government to release the data it reviewed before licensing Pfizer’s COVID-19 vaccine. In response, the Food and Drug Administration (FDA) proposed it be given 55 years to review and produce its vaccine-related documents. (The timeline for the release of the FDA’s documents is still being determined in the courts).
Thanks to the team of 30 scientists and professors who filed suit in September, the first round of the FDA’s documents have been released. Though it’s only 91 of the 329,000 pages sought by the plaintiffs, the public can view the documents on the Public Health and Medical Professionals for Transparency’s (PHMPT) website.
A point of interest from the released documents, as noted by Aaron Siri on Substack, is the revelation that only two and a half months after the FDA granted Emergency Use Authorization (EAU) for the Pfizer-BioNTech COVID-19 vaccine, Pfizer had to make “workflow solutions” due to large amounts of adverse events.
In a Pfizer document titled “Cumulative Analysis of Post-authorization Adverse Event Reports received through 28-FEB-2021,” it is indicated that Pfizer was overwhelmed by the reports of adverse vaccine reactions.
On page 6, the report reads that “Due to the large numbers of spontaneous adverse event reports received for the product, [Pfizer] has prioritised the processing of serious cases, in order to meet expedited regulatory reporting timelines.”
“Pfizer has also taken a [sic] multiple actions to help alleviate the large increase of adverse event reports. This includes significant technology enhancements, and process and workflow solutions, as well as increasing the number of data entry and case processing colleagues,” the report continued before noting the redacted number of new employees they had hire.
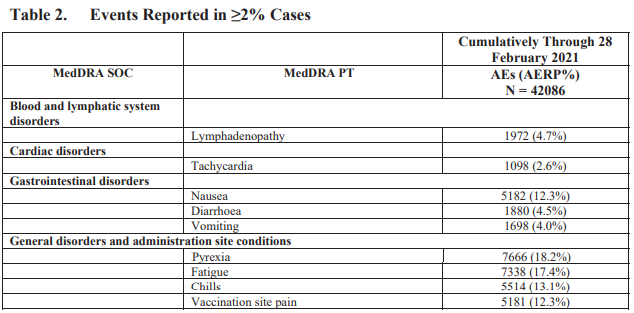
According to the same document, from December 1, 2020 through the end of February (90-day period), “there was a total of 42,086 case reports […] containing 158,893 events.” Of those events, 25,957 fell under the “Nervous system disorders” category.
Another point of concern from this report is that women between the ages of 31 and 50 were the most likely to experience vaccine adverse events. As shown in the table below, 29,914 events were provided by women vs. 9,182 by men. What’s more, there were 13,886 relevant cases from 31 to 50-year-olds vs. the 5,214 from the most at-risk demographic, individuals 75 years and older.
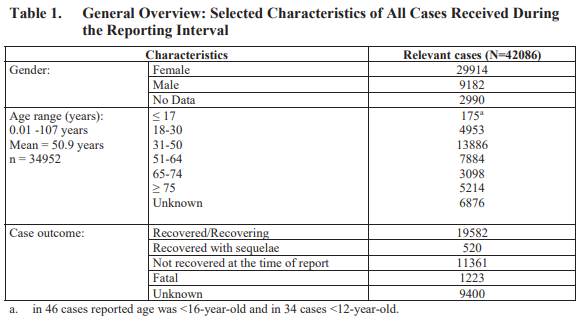
The most alarming discovery from the report is that of the 42,086 adverse event cases, 1,223 cases (or 2.90%) were fatal. Overall, the report stated more women with Pfizer vaccine adverse events, it did not provide the proportion of fatalities by gender.
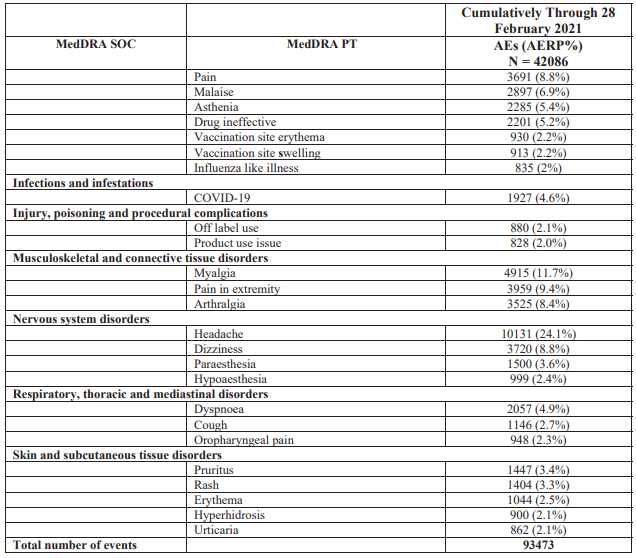
The most common Pfizer vaccine adverse events (multiple vaccine adverse events can be associated with a single case) in the report were: headache (24.1%), pyrexia or fever (18.2%), fatigue (17.4%), chills (13.1%), nausea (12.3%) and vaccination site pain (12.3%).
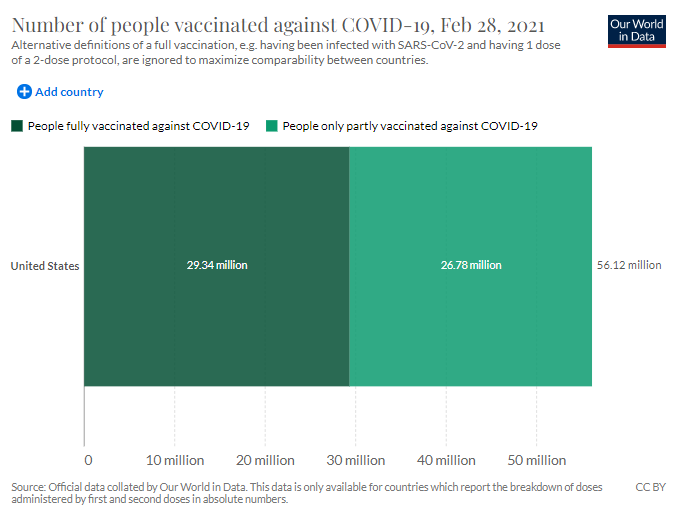
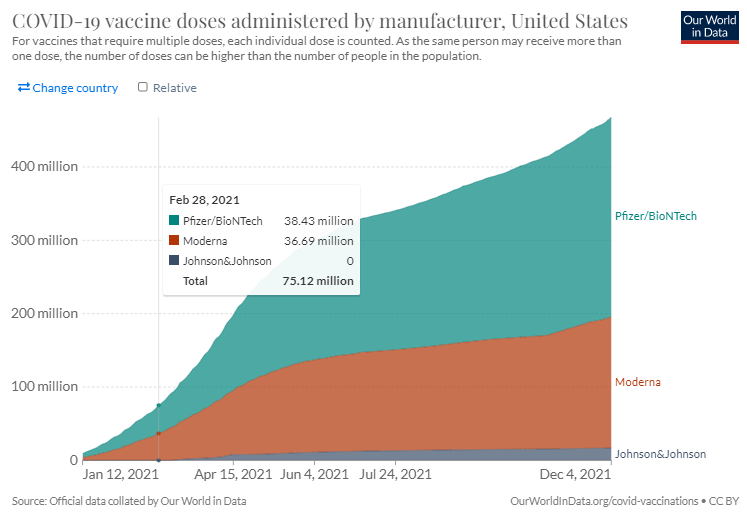
According to Our World in Data, during the period stated in the report, there were a total of 75.12 million COVID-19 vaccine doses administered to approximately 56.12 million people in the United States. The data shows approximately 38.43 million doses administered were that of the Pfizer vaccine during the same period as the report.
On page 24 of the report, Pfizer makes it sound as if the scope and frequency of these reactions were to be expected. “The findings of these signal detection analyses are consistent with the known safety profile of the vaccine,” the document states. But as Aaron Siri noted, “if they knew these issues were going to arise, then why didn’t they appear to have enough staff to process this expected volume of reports?”
The report’s conclusion reads, “The data do not reveal any novel safety concerns or risks requiring label changes and support a favorable benefit-risk profile of to the BNT162b2 vaccine.”
The PHMPT, the team of scientists and professors who were instrumental in making this document publicly available, filed a second-joint report mid-November at the Norther District of Texas U.S. Court. In their report, the PHMPT make a case for expediting the process of releasing the rest of the FDA’s documents.
“The entire purpose of the FOIA is to assure government transparency. It is difficult to imagine a greater need for transparency than immediate disclosure of the documents relied upon by the FDA to license a product that is now being mandated to over 100 million Americans under penalty of losing their careers, their income, their military service status, and far worse,” the plaintiffs argue.
“It took the FDA precisely 108 days from when Pfizer started producing the records for licensure on May 7, 2021,14 to when the product was licensed on August 23, 2021. We assume, as the FDA has stated, that it conducted an intense, robust, thorough and complete review and analysis of those documents in order to assure that the Pfizer vaccine was safe and effective for licensure. The FDA now has an equally important task of making those documents available to the Plaintiff in this case and the public at large in at least the same timeframe.”
“The FDA’s own regulations envision and reflect upon the importance of making this information public as soon as a vaccine is licensed. Its regulations provide that it is to make ‘immediately available’ all documents underlying licensure of a vaccine. The FDA knew the intense public interest in that data and information. It should have been preparing to release it simultaneously with the licensure,” the filing concludes. “Instead, it has done the opposite.”
Related Articles
Author: Bo John Brusco














3 Responses
The 2,9% is not right. I suppose that it relates to ca. 140.000.000 vaccins.
So that is 0,0008%.
And 42.000 injuries = ca. 0,03%.