On August 23, the Food and Drug Administration granted approval for a COVID-19 vaccine. But the jab in question is called the “Comirnaty” vaccine. The labels for vials of Pfizer-BioNTech are still marked for “Emergency Use Authorization.”
The Food and Drug Administration’s (FDA) approval of a COVID-19 vaccine has encouraged elected officials to move forward with vaccine mandates. Many government employees must now choose between vaccination or termination.
While Gov. Jay Inslee enacted his proclamation for state employees before the FDA’s approval, President Joe Biden recently expanded vaccine requirements at the federal level. Before issuing the new mandate, President Biden noted how some Americans have been vaccine-hesitant because the FDA hadn’t approved a COVID-19 vaccine until recently.
“Many said they were waiting for approval from the Food and Drug Administration,” he said during his September 9 address. “Well, last month, the FDA granted that approval.”
To read the Executive Order signed by President Biden mandating coronavirus vaccination for all Federal employees, click here.
What was and was not approved
On August 23, the FDA did grant its approval for a COVID-19 vaccine, but potentially not a labeled FDA-approved vaccine that is currently available for distribution. While news outlets have reported as though the Pfizer vaccine received FDA approval, like the New York Times’ headline, “F.D.A. Fully Approves Pfizer-BioNTech’s Vaccine, a First for a Covid-19 Shot,” the vaccine that is officially approved is called “COMIRNATY® (COVID-19 vaccine, mRNA).”
This information is publicly available. The approval announcement on the FDA’s website reads, “On August 23, 2021, the FDA approved the first COVID-19 vaccine. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty, for the prevention of COVID-19 disease in individuals 16 years of age and older.”
Concerning this new marketing change, on August 23, the FDA stated in a letter to Pfizer that “the licensed vaccine (Comirnaty) has the same formulation as the EUA-authorized vaccine (Pfizer-BioNTech) and the products can be used interchangeably to provide the vaccination series without presenting any safety or effectiveness concerns.”
In other words, a pharmacist is permitted (depending on the laws of a state) to substitute an interchangeable product (Pfizer-BioNTech COVID-19 Vaccine) for the reference product COMIRNATY® (COVID-19 Vaccine, mRNA) without consulting the prescriber—a practice commonly called pharmacy-level substitution.
While the document claims that the two vaccines share the same formulation, the letter states in its footnotes that “the products are legally distinct with certain differences that do not impact safety or effectiveness” (emphasis added).
It is also clear that while the FDA states that the two formulations can be used interchangeably, the Pfizer-BioNTech vaccine remains under the Emergency Use Authorization while the COMIRNATY® (COVID-19 Vaccine, mRNA) is FDA approved.
This fact is reiterated throughout the FDA’s documentation. In their fact sheet for recipients and caregivers, for example, it reads, “The FDA-approved COMIRNATY (COVID-19 Vaccine, mRNA) and the FDA-authorized Pfizer-BioNTech COVID-19 Vaccine under Emergency Use Authorization (EUA) have the same formulation and can be used interchangeably to provide the COVID-19 vaccination series.”
This is further emphasized with the statements “COMIRNATY® (COVID-19 Vaccine, mRNA) is an FDA-approved COVID-19 vaccine made by Pfizer for BioNTech” and “Pfizer-BioNTech COVID-19 Vaccine has received EUA from FDA” in Pfizer’s latest press release announcing collaboration with Brazil’s Eurofarma to manufacture COVID-19 vaccine doses.
This press release by Pfizer went on to state that, “This emergency use of the product has not been approved or licensed by FDA, but has been authorized by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19)…”
In a September 6 press release announcing a submittal to a request by the European Medicines Agency (EMA) to update its Conditional Marketing Authorization (CMA) for a booster dose, BioNTech, Pfizer’s co-partner in the production of the Pfizer-BioNTech COVID-19 vaccine, clearly states, “The Pfizer-BioNTech COVID-19 vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19)…”
This fact is also indicated in the product’s labeling. In FDA’s previously mentioned letter to Pfizer, it states that “The Pfizer-BioNTech COVID-19 Vaccine vial label and carton labels are clearly marked for ‘Emergency Use Authorization.’”
The Lynnwood Times reached out to the FDA to clarify why the Pfizer-BioNTech COVID-19 Vaccine is being marketed as the Comirnaty when, according to the FDA’s own letter to Pfizer, they are legally “distinct” and appear to be two different vaccines. The Times also questioned why the vaccines can be used interchangeably when the “COMIRNATY® (COVID-19 Vaccine, mRNA)” is FDA-approved and the Pfizer-BioNTech COVID-19 vaccine is only authorized. As of Tuesday, September 14, the FDA has not responded to these inquiries.
Do Americans have access to the only FDA-approved vaccine?
Perhaps the most consequential difference between these vaccines is their current availability. “Although COMIRNATY (COVID-19 Vaccine, mRNA) is approved to prevent COVID-19 in individuals 16 years of age and older, there is not sufficient approved vaccine available for distribution to this population in its entirety at the time of reissuance of this EUA,” reads one of the footnotes on page 5 in the FDA’s letter.
Hence, this is a possible reason the FDA is allowing the FDA-authorized Pfizer-BioNTech COVID-19 Vaccine under Emergency Use Authorization to be used interchangeably with FDA-approved COMIRNATY® (COVID-19 Vaccine, mRNA).
Admittedly, the language is vague; it is difficult to say for certain what specific population the statement refers to and what “sufficient” exactly entails. Seeking clarification, the Lynnwood Times reached out to the FDA for comment regarding the availability of COMIRNATY® (COVID-19 Vaccine, mRNA) to American citizens. As of Tuesday, September 14, the FDA has yet to respond.
In a response to Lynnwood Times’ inquiry to when Washington state can expect to receive its first shipments of COMIRNATY® (COVID-19 Vaccine, mRNA), Shelby Anderson, Public Information Officer for the Washington State Department of Health, shared that the vaccine is currently available for use.
“The vaccine is available now in Washington, as it is the same formulation as that under EUA. Providers will continue to use and order the same stock as always so they already have it in their freezers.”
Yet, it is unclear if the re-ordered vaccine will be labeled with FDA-approved COMIRNATY® (COVID-19 Vaccine, mRNA) or the FDA-authorized Pfizer-BioNTech COVID-19 Vaccine. Vaccines that are currently labeled in freezers being administered to Washingtonians are the Pfizer-BioNTech COVID-19 Vaccine, Moderna COVID‑19 Vaccine, and the Johnson & Johnson’s Janssen COVID-19 Vaccine, all of which are currently under an FDA Emergency Use Authorization.
Vaccine Interchangeability
The FDA repeatedly states how the Pfizer-BioNtech and Comirnaty vaccines can be used interchangeably. According to the FDA’s website, for a product to be interchangeable with another, it must be “biosimilar to a reference product” and “be expected to produce the same clinical result as the reference product in any given patient” with “no additional risk or reduced efficacy if a patient switches back and forth between an interchangeable product and a reference product.”
AstraZeneca, whose vaccine has been issued for use in other countries, explains on its website that “the steps within the production of similar vaccines, that have the same mode of action, may vary between manufacturers. This may mean that vaccines could contain different concentrations of their active substance, different preservatives, or different stabilisers.”
An online database called “Purple Book” by the FDA contains information on FDA-licensed (approved) biological products regulated by the Center for Drug Evaluation and Research, including licensed biosimilar and interchangeable products, and their reference products.
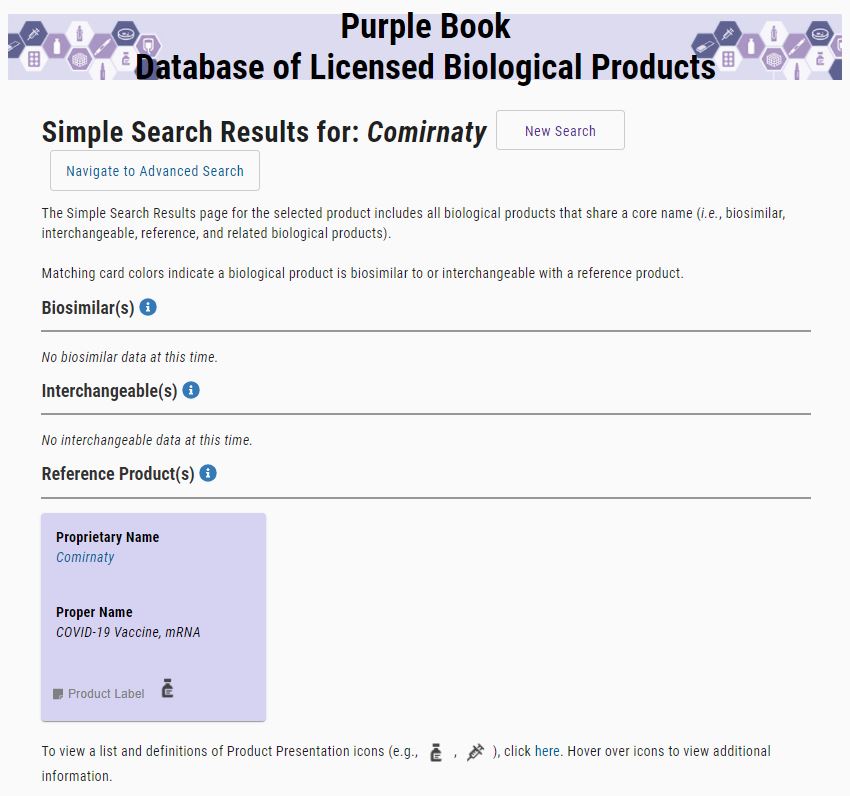

Of all the COVID-19 vaccines we queried, COMIRNATY® (COVID-19 Vaccine, mRNA) is the only one to produce a result. According to the Purple Book, there is no FDA-licensed (approved) interchangeable product for COMIRNATY® (COVID-19 Vaccine, mRNA). This adds further evidence to the fact that the Pfizer-BioNTech COVID-19 Vaccine is FDA-authorized, meaning it is under Emergency Use Authorization.
The Lynnwood Times is awaiting clarification from the FDA if an FDA “approval” for one vaccine is transferable to another vaccine if that other vaccine is under an EUA status, deemed interchangeable, and states to have the same formulation.
Emergency Use Authorization and Vaccine Mandates
Liberty Counsel, a national nonprofit litigation public policy organization, stated in an April 2021 memo that FDA-authorized COVID vaccines cannot be mandated nor required as a condition of employment because of its Emergency Use Authorization (EUA) categorization by the Food and Drug Administration (FDA).
To view the memo on COVID Vaccine Mandates from Liberty Counsel click here.
In a memo from Liberty Counsel, it states, “COVID vaccines are in a special category and cannot be treated like FDA licensed vaccines. None of the COVID vaccines are FDA licensed; nor have they received full FDA approval. Rather, their approval is under the special provision noted above as EUA. This means that there is not enough data (which includes duration of testing) for the FDA to render a final approval. More importantly, no one, including private employers, may coerce individuals (by threatening their employment or otherwise) to take an EUA vaccine. Federal law requires full and informed, voluntary consent.”
The memo further states that under 21 U.S.C. §360bbb-3, individuals — whether employed by religious organizations, or not — have “the option to accept or refuse administration of the product” and that the “FDA has an obligation to ensure that recipients of the vaccine under an EUA are informed… that they have the option to accept or refuse the vaccine.”

In an August 2020 meeting by the Centers for Disease Control Advisory Committee on Immunization Practices, Executive Secretary Capt. Amanda Cohn, MD, confirmed the non-mandatory nature of an EUA vaccine: “under an Emergency Use Authorization, an EUA, vaccines are not allowed to be mandatory.”
Employers are prohibited by federal law to single out employees by questioning their COVID status or requiring screening unless there is reasonable belief the employee has the disease. The U.S. Equal Employment Opportunity Commission has also stated that employers cannot inquire about family members of an employee who may have COVID or COVID-like symptoms.
According to the FDA, EUA “may allow the use of unapproved medical products, or unapproved uses of approved medical products in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, including that there are no adequate, approved, and available alternatives.”
Dr. William Moss, the executive director of the International Vaccine Access Center at Johns Hopkins Bloomberg School of Public Health, spoke about some of the differences between EUA and full approval in an interview with ABC News.
Moss told ABC that while “both authorization and approval are rigorous processes that look at the safety and efficacy of a vaccine,” time is a key factor. Authorization requires “at least two months of follow-up data from Phase 3 clinical trials” whereas approval needs at least six months of follow-up.
Transparency and Trust
The linguistic ambiguity surrounding this subject is pervasive. As the Comirnaty and Pfizer-BioNTech COVID-19 vaccines are both made by BioNTech and Pfizer, conflating the EUA vaccine with the approved one is concerningly easy. According to Pfizer’s website, the Comirnaty vaccine is “made by Pfizer for BioNTech.” Both could be referred to as the Pfizer vaccine or even BioNTech’s vaccine.
Going back to Biden’s remarks and the FDA’s official statement of approval, the language is careful to state that a COVID-19 vaccine has been FDA approved without directly connecting that approval status with the Pfizer-BioNTech vaccine.
The increasing polarization among political tribes is coinciding with a rapid decline of trust in government entities. While arguments surrounding collective safety and individual freedom are essential, their ability to be constructive is predicated on clear answers and transparent communication.
With this duty to truth in mind, the Lynnwood Times will continue to follow this story and report new developments as they come.
Primary author for this article is Bo John Brusco with some contributions from Lynnwood Times Publisher Mario Lotmore.



















21 Responses
Excellent article. I appreciate the homework you are doing on this subject. This information essentially suggests that Feds, states, and employers who are saying get the vaccine or get fired are actually doing something that is illegal–since I don’t believe there is any inventory of Comirnaty in WA state. Someone needs to call Inslee out on this one.
I believe that once an approved vaccine is readily available, emergency use for any non-approved vaccine cannot happen.
“Readily available”
Also, they will never “approve, available,” etc either HCQ or IVM, as that will blow all vaxxes out of the water. We would not need any of these dangerous jabs if we could use the early treatments that actually work. Just ask India.
Thank you for all the research dedicated to this article. I appreciate the fact of references used in all of your articles. If I want to find the information and research myself I am able to do so easily with the references.
Thanks for this work. It’s real clear, there is deception at play. In the light of truth, this kind of carefully crafted wording, smoke and mirrors, etc. is simply not required. What this is about is pushing folks to get vaccinated under ostensible approval, while continuing to shield the pharmaceutical companies from liability. It’s not more complicated that that, period.
John 3:21 But whoever lives by the truth comes into the light, so that it may be seen plainly that what they have done has been done in the sight of God.
You missed the main point. The feds want approval to back up their vaccine mandates but that opens the vaccine to lawsuits. Hence the legal distinction is the the inclusion of side affects myocarditis and pericarditis and the age limit raised to 16. Notice the comirnaty manufacturer is no longer Pfizer BioNtech but BioNtech. Try and sue a foreign company!
The author certainly put lots of time and effort into this piece. However, I am surprised that the Lynnwood Times would be bothering to try to discover some grand conspiracy at work with this. I think the author misses the point completely, in that a legal distinction or different preservatives being used in one medication and one labeling does not make it different in efficacy or safety. The EUA is still in effect for some uses, not to mention, that legally they have to make the point that these vaccinations that have different labels are under different authorizations.
It’s not some conspiracy and you haven’t even interpreted the language accurately. I would recommend looking at this page on the FDA’s website to get accurate and easy to read information: https://www.fda.gov/vaccines-blood-biologics/qa-comirnaty-covid-19-vaccine-mrna.
The tl:dr version from the FDA:
Is Comirnaty interchangeable with other COVID-19 vaccines?
Comirnaty has the same formulation as the FDA-authorized Pfizer-BioNTech COVID-19 vaccine and can be used interchangeably to provide the COVID-19 vaccination series without presenting any safety or effectiveness concerns. The products are legally distinct with certain differences that do not impact safety or effectiveness.
I would think the Lynnwood Times wouldn’t want to traffic in misinformation, but, here we are.
I’ve read this article twice and shared it. This information is so valuable. It has been difficult to put all the pieces together for so many people, but this has made it very clear that the emergency approved vaccine cannot be forced on us, we cannot be threatened with termination, or required to be tested without reason.
We are clearly being deceived, and I appreciate that Bo has the courage and intelligence to report the truth. People need to be informed, rather than blindly trusting those taking control and complying out of fear.
Thank you Bo!
Hi there very nice site!! Guy .. Excellent ..
Superb .. I’ll bookmark your site and take the feeds also?
I am happy to find so many useful information here in the put
up,
I have had a hard time clearing my mind in getting my thoughts out.